 Introduction
Introduction
 Physics Today paper
Physics Today paper
 Intro
Intro
Your opponent's serve was almost perfect, but you vigorously returned it beyond his outstretched racquet to win the point. Now the tennis ball sits wedged in the chain-link fence around the court. What happened to the ball's kinetic energy? It has gone to heat the fence, of course, and you realize that if the fence were quite a bit colder, you might be able to measure that heat and determine just how energetic your swing really was.
Calorimetry has been a standard measurement technique since James Joule and Julius von Mayer independently concluded, about 150 years ago, that heat is a form of energy. But only in the past 15 years or so has calorimetry been applied, at millikelvin temperatures, to the measurement of the energy of individual photons and particles with exquisite sensitivity.
Low-temperature microcalorimeters have several unique properties that have led to a variety of applications.1 Here, to begin, we introduce a few of those applications before focusing in detail on high-resolution astrophysical x-ray spectroscopy. Still relatively young, the technique of quantum calorimetry (the thermal measurement of energy quanta) will no doubt find more applications that will be just as exciting and potentially revolutionary as the advances promised in x-ray astronomy.
Because of the exceedingly small specific heats of many materials at very low temperatures, detectors can be quite large and still be sensitive to small amounts of deposited energy. This energy is sensed after it has been converted to heat, so that even interactions that produce little or no ionization can be detected.
These two properties have made calorimetry an attractive choice in searches for WIMPs, the weakly interacting massive particles that are among the leading candidates for the missing dark matter in the universe. The scattering of a single WIMP is predicted to transfer roughly 1 keV to nuclei of the target material. Such slow nuclei produce much less ionization than electrons of the same energy. By simultaneously measuring both the thermal and ionization signals, it is possible to efficiently glean the real events from the much more numerous background events that interact with electrons and produce relatively more ionization.2
This sensitivity to nonionizing events can also be used to detect DNA fragments in a time-of-flight mass spectrometer. At energies of 10--20 keV, these massive molecules produce little or no ionization on impact. Nevertheless, the energy resolution of a thermal detector is ample for distinguishing singly and doubly charged fragments.3
Another useful property of thermal detection is that it does not depend on the charge transport properties of the absorber. Only a very few materials, among them highly pure silicon or germanium, can be used to make ionization detectors, whereas a calorimeter can even incorporate the radioactive source being investigated.4 This advantage is being exploited in investigations of beta decay spectra to put limits on the neutrino rest mass. Beta spectrometer results are limited by the systematic uncertainties that arise when correcting for energy lost in the source. By contrast, a calorimeter that incorporates the source measures directly the total decay energy minus whatever is carried away by the neutrino --- provided no metastable states are created with lifetimes longer than the thermal integration time of the detector.5
When a calorimeter measures energy, it is in a
low-temperature equilibrium state in which nonthermal
excitations have negligible populations. To the extent
that a calorimeter can be regarded as a closed system,
the event-to-event statistical fluctuations that limit
the resolution of an ionization detector do not occur.
It is possible to make small calorimeters with resolving
power 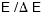 of 5000 or
more for x-rays ---
almost a factor of 100 better than an ideal silicon solid-state
detector and a great leap forward not just for x-ray astronomy,
but also for materials microanalysis. (See PHYSICS TODAY,
July 1998, page 19.)
of 5000 or
more for x-rays ---
almost a factor of 100 better than an ideal silicon solid-state
detector and a great leap forward not just for x-ray astronomy,
but also for materials microanalysis. (See PHYSICS TODAY,
July 1998, page 19.)
Lower, but still useful, resolving power can be extended all the way down to the near infrared, raising the possibility of fabricating imaging arrays for visible light that simultaneously provide spectra and photometry.6
Since much of the early work on quantum calorimeters was inspired by a quest for better astrophysical x-ray spectrscoopy, we use this application as a detailed example of the basic physics of calorimeters. But the results are general, and can also be used to determine the suitability of calorimetric methods for other experiments.
|