 Introduction
Introduction
 Physics Today paper
Physics Today paper
 The basics
The basics
As shown in figure 2, an x-ray calorimeter is conceptually a simple device, whose basic principles are obvious from the outset. Those principles are as follows:
How well can a calorimeter measure the energy it absorbs?
Consider a calorimeter with a thermometer that translates
changes in temperature into a voltage. It has a heat capacity
C and a link with thermal conductance G to a heat sink.
An instantaneous deposition of energy into the absorber
produces a voltage pulse with an exponential decay time
constant 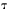 equal to C/G.
equal to C/G.
In the frequency domain, the signal is nearly independent of
frequency f when f<< 1/2 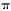
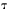 ,
and falls off as 1/f above this corner frequency.
The magnitude of the noise due to the random transfer of
energy across the link to the heat sink (an elementary
calculation in statistical mechanics) has the same frequency
distribution as the signal. If this so-called phonon noise
were the only source of noise, the signal-to-noise ratio
would not depend on frequency, and the measurement error
could be made arbitrarily small by employing a sufficiently
large bandwidth.
,
and falls off as 1/f above this corner frequency.
The magnitude of the noise due to the random transfer of
energy across the link to the heat sink (an elementary
calculation in statistical mechanics) has the same frequency
distribution as the signal. If this so-called phonon noise
were the only source of noise, the signal-to-noise ratio
would not depend on frequency, and the measurement error
could be made arbitrarily small by employing a sufficiently
large bandwidth.
In reality, a frequency-independent noise term and a finite detector response time both reduce the signal-to-noise ratio at high frequencies, effectively limiting the usable bandwidth. This state of affairs is illustrated in figure 3.
To understand the bandwidth limitations and calculate the highest energy resolution attainable, we need to consider specific implementations of the calorimeter concept, which, to date, have been based on resistive, capacitive, inductive, paramagnetic, and electron tunneling thermometers. The best energy resolution so far has been achieved with resistive thermometers --- specifically, semiconductor thermistors and superconducting transition-edge sensors.
Johnson noise and Joule heating are important effects in resistive calorimeters. An electrical analog of Brownian motion, Johnson noise is frequency-independent voltage noise that scales with the square root of temperature and resistance. Joule heating occurs because the bias current or voltage used to convert a change in resistance to an electrical signal is continually dissipating power in the thermometer. (See figure 4 for representative circuit diagrams illustrating the role of the applied bias.)
These two physical effects lead to the concept of optimal bias. Increasing the bias raises the signal and phonon noise relative to the Johnson noise, extending the useful bandwidth, until the decrease in signal-to-noise resulting from self-heating offsets that gain. Also contributing to the optimization is the increase in heat capacity with temperature.
Fifteen years ago, Harvey Moseley (the US father of quantum
calorimetry) and his colleagues7
calculated the energy resolution attainable in an ideal calorimeter with a
resistive thermometer, following the similar optimization
done by John Mather for infrared
bolometers.8
Moseley obtained an expression for the energy resolution of the form
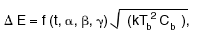
where kis Boltzmann's constant, Tb
is the temperature of the heat sink, and Cb
is the heat capacity evaluated at that temperature.
Writing the reduced temperature T/Tb as t,
the heat capacity and thermal conductance functions are
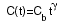
and
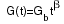
respectively, but the
thermal conductance does not appear in the expression for
 except through its exponent
except through its exponent
 .
.
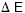 depends on the resistance through the logarithmic
sensitivity
depends on the resistance through the logarithmic
sensitivity 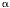 , which is defined by
, which is defined by
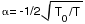
For simplicity, we have not included the definition of
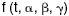 above, but, for most practical
values of
above, but, for most practical
values of 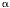 and
and 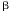 ,
,
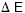 is proportional to
is proportional to
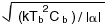 .
Minimizing the full expression in t determines the optimal
biastemperature and the highest resolution for a given
detector.
.
Minimizing the full expression in t determines the optimal
biastemperature and the highest resolution for a given
detector.
It is assumed that an incident photon changes the temperature
so little that it is valid to neglect the temperature
dependence of C, G, 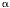 , and the
noise terms during the pulse.
External sources of noise, such as from a signal amplifier, are also
assumed to be negligible compared with the intrinsic noise.
Though amplifier noise requires careful attention, it can be
rendered negligible for resistive thermometers by the right
choice of detector resistance and by the use of low-noise
junction field effect transistors (JFETs) for high-impedance
devices and superconducting quantum interference devices
(SQUIDs) for low-impedance devices.
, and the
noise terms during the pulse.
External sources of noise, such as from a signal amplifier, are also
assumed to be negligible compared with the intrinsic noise.
Though amplifier noise requires careful attention, it can be
rendered negligible for resistive thermometers by the right
choice of detector resistance and by the use of low-noise
junction field effect transistors (JFETs) for high-impedance
devices and superconducting quantum interference devices
(SQUIDs) for low-impedance devices.
Another property of resistive thermometers, electrothermal
feedback (ETF), occurs because the bias power changes as the
resistance changes. Consider a thermistor with a negative
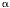 that is biased with a constant
current I,
as in the first example in figure 4.
As T increases, R will fall, causing
I2R, the Joule power, to drop.
This loss makes the pulse recovery time faster than the value of
C/G expected in the absence of feedback.
In devices with large
that is biased with a constant
current I,
as in the first example in figure 4.
As T increases, R will fall, causing
I2R, the Joule power, to drop.
This loss makes the pulse recovery time faster than the value of
C/G expected in the absence of feedback.
In devices with large 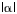 ,
this effect can be very important.
,
this effect can be very important.
|