 Introduction
Introduction
 Details
Details
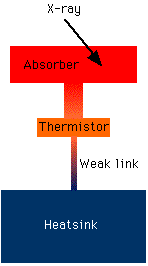 Here are some more details of how calorimeters work:
Here are some more details of how calorimeters work:
X-rays are a form of electromagnetic radiation, like visible light, ultraviolet and infrared light, gamma rays or radio waves. And electromagnetic radiation comes in packets called photons. Each photon carries a certain amount of energy, which is what we are trying to measure.
Heat is a form of energy. In our detectors, the energy of the X-ray is converted completely to heat, so by measuring the amount of heat we are measuring the energy of the X-ray.
Actually, it isn't always true that all the energy of the X-ray is converted to heat. Some of the energy can be lost by various means, and when this happens the energy converted to heat is less than the true energy of the X-ray. This is a form of error in the detectors, and we work hard to minimize and characterize it.
An X-ray which hits the absorber knocks an electron loose from an atom of the absorber material. This photoelectron then rattles around in the absorber, colliding with other atoms and losing energy (which is converted to heat). When everything has settled down (after a few microseconds), the energy has all been converted to heat.
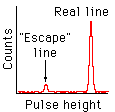 Sometimes the initial photoelectron escapes from the detector, taking some
energy with it. This makes the measured
energy too small by however much the electron carries away.
Sometimes the initial photoelectron escapes from the detector, taking some
energy with it. This makes the measured
energy too small by however much the electron carries away.
Because the ionization energy of any element is fixed, many of these incorrect measurements will be wrong by the same amount. Thus any real feature in the X-ray spectrum we are trying to measure will have an "escape" copy at a lower energy, as shown here. Fortunately this only happens about 1% of the time.
To measure the X-ray energy accurately, we need to measure the temperature of the detector accurately. The first step to doing this is to make sure that the detector actually has a temperature. That is, it needs to be in what we call thermodynamic equilibrium.
When something is in thermodynamic equilibrium, its molecules are vibrating with a specific distribution of speeds. Some molecules vibrate faster than others, but at a given temperature there is a certain fraction vibrating with any given speed. Any object will eventually end up in thermodynamic equilibrium.
Now, when a photon is absorbed by the absorber, it starts off with a non-equilibrium distribution of molecular speeds. Specifically, too many of the molecules are vibrating especially fast. If these vibrations get to the thermistor, it will respond as if the absorber were at a higher temperature, giving us the wrong value for the X-ray energy.
However, the isolation between absorber and thermistor gives the absorber time to come into equilibrium before the thermistor starts to heat up. That way, the thermistor responds to the true temperature of the absorber, and hence the true energy of the X-ray.
In order for the X-ray to cause a measurable change in temperature, the heat capacity of the detector must be kept small. (That should be obvious; a single X-ray photon absorbed by, say, a bowling ball will not raise its temperature much at all!)
 As an example let's calculate how much an X-ray photon
would raise the temperature of a penny at room temperature.
Modern U.S. pennies
consist of 2.5 grams of zinc with a thin copper plating, and
zinc has a specific heat
of .38 Joules/gram-Kelvin, so a penny has a heat capacity C of about 1 Joule/Kelvin (1
Kelvin is 1 degree Celcius &emdash; the only difference is the Kelvin scale
starts at absolute zero).
As an example let's calculate how much an X-ray photon
would raise the temperature of a penny at room temperature.
Modern U.S. pennies
consist of 2.5 grams of zinc with a thin copper plating, and
zinc has a specific heat
of .38 Joules/gram-Kelvin, so a penny has a heat capacity C of about 1 Joule/Kelvin (1
Kelvin is 1 degree Celcius &emdash; the only difference is the Kelvin scale
starts at absolute zero).
A 6keV X-ray has an energy E of about 10-15Joules, so it will raise the penny's temperature by
dT = E / C
= 10-15 Kelvin (0.000000000000001 Kelvin)
Since room temperature is about 300 Kelvin, that is a fractional change of 10-15 / 300, or about 3 parts in 1018. This is far beyond any temperature measuring capability devised by humans. (In fact it is beyond mankind's ability to measure anything!)
Now let's consider 1 gram of silicon at 0.1 Kelvin. At that low temperature, the heat capacity becomes extremely small &emdash; 2.6×10-10 Joules/Kelvin. Then a 6keV X-ray photon will cause a temperature rise of about 4 microKelvin. Dividing by 0.1 Kelvin gives a fractional change of 4 parts in 105. This is a range that can be measured. Of course, our detectors each contain much less than one gram of silicon, giving a much larger fractional temperature change.
Naturally, there are additional complications. For instance, materials
with such low heat capacity are generally not good X-ray absorbers,
so we have to trade off between absorbing more X-ray photons and keeping
the heat capacity low.
Our thermistors are basically the same kind of silicon semicondoctor that you find inside your computer. We inject impurities into pure silicon, which allows it to conduct electricity. The amount it conducts depends on the amount of impurity we add, and on the temperature. With the right level of impurity, we can make resistors that change their resistance very rapidly around 60 milliKelvin.
Of course, there are other ways to measure temperature. One of the most promising is to use a superconductor right at the temperature where it changes from being normal to superconducting. At that precise temperature, the resistance changes extremely rapidly with temperature, giving us a very accurate way to measure the temperature. This is known as a Transition-Edge Sensor (TES), and several of us are developing this type of sensor.
Below is a schematic drawing of a detector. The absorber is glued to the top of a small silicon attachment block, which in turn is glued to the silicon detector body. This serves as the weak link between the absorber and the thermistor.
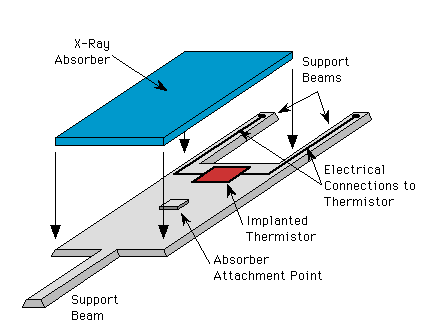
Schematic drawing of a detector.
The weaker link between the detector and the heat sink is through the three support legs. The legs are arranged this way to allow two rows of detectors to be placed next to each other, with the single legs going between pairs of detectors in the opposing row (see photos below).
The detectors are manufactured as a monolithic array, all 32 at once. Like a sculptor, we start with a wafer of silicon, and chemically etch away everything that doesn't look like detectors. The thermistor and its electrical connections are then made by implanting impurities into the silicon. Finally, the attachment blocks are glued to the detectors, and the absorbers are glued onto the blocks. This gluing is done by hand (under a microscope).
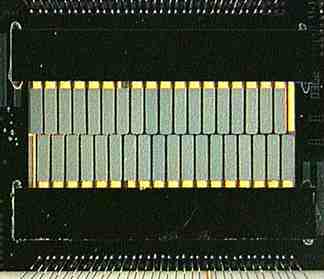 This is a photo of a detector array from an
XQC rocket
flight. It contains 32 detectors in 2 rows of
16. The detectors (actually the mercury-telluride absorbers)
are the gray-green rectangles in the middle.
This is a photo of a detector array from an
XQC rocket
flight. It contains 32 detectors in 2 rows of
16. The detectors (actually the mercury-telluride absorbers)
are the gray-green rectangles in the middle.
Most of the length of each detector's legs is covered by a strip of silicon, which shows up black in this photo. When an X-ray is absorbed by a detector leg, the energy is not completely converted to heat before it gets to the thermistor. For this reason, the legs are covered.
The leg covers don't completely cover the detectors' legs. Where the legs are open, you can see through the spaces between them to the gold plated box in which the detectors are mounted.
At the top and bottom of the photo are the bonding wires which connect
the detector to the outside world.
 Here is a closeup of the array. You can (barely) see two black legs
from each detector at the top of the image, and one leg from each detector
squeezing between a pair of detectors at the bottom.
Here is a closeup of the array. You can (barely) see two black legs
from each detector at the top of the image, and one leg from each detector
squeezing between a pair of detectors at the bottom.
Between the detector legs, the gold plated box is clearly visible.
|
This page written and maintained by
Kevin R. Boyce
(email: Kevin.R.Boyce@gsfc.nasa.gov)
This page was last modified on Wednesday, 22-Nov-2000 10:15:28 EST